Det finns mer bakom okontrollerad astma
DUPIXENT går längre än att bara påverka EOS genom att angripa typ 2-inflammation1-3
| MER PRECISION Den enda biologiska behandlingen som specifikt riktar in sig på både IL-4 och IL-13 för att stoppa lokal och systemisk typ 2-inflammation vid källan1-3,a |
MER MÖJLIGHETER Förbättringar som dina patienter kan känna, och varaktigt skydd mot sjukdomsprogression som du kan se1,4,b |
| Utforska mer | Lär mer |
up to
up to
up to
up to
a Systemic eosinophilic and allergic inflammation and local airway inflammation.
b Improvement in FEV1 at Week 2 (primary endpoint was Week 12); sustained through Week 52 (QUEST) and ~3 years (TRAVERSE OLE); exacerbation reduction through Week 52 (QUEST); reduced FeNO, mucus plugging, and airway resistance and increased airway volume at Week 24 (VESTIGE).
c 550 mL improvement in FEV1 at Week 52 with DUPIXENT 200/300 mg + SOC (n=69) in patients with EOS ≥300, FeNO ≥25, and allergic asthma on high-dose ICS. Only descriptive statistics (no P-values) are available for data due to the single-arm nature of the open-label extension study.
d 75% reduction in annualized severe exacerbations at Week 52 with DUPIXENT 200/300 mg + SOC (n=91) in patients with EOS ≥300, FeNO ≥25, and allergic asthma on high-dose ICS. Only descriptive statistics (no P-values) are available for data due to the single-arm nature of the open-label extension study.
e 86% of patients reduced or eliminated OCS use by Week 24 with DUPIXENT 300 mg + SOC (n=103) vs 68% with placebo + SOC (n=107) (P<0.001).
f Prebronchodilator FEV1, P nominal <0,001
SE bifogad annons1
Dupixent är indicerat för vuxna och ungdomar (12 år och äldre), som tillägg till underhållsbehandling vid svår astma med typ 2 inflammation, som kännetecknas av förhöjda nivåer av eosinofiler och/eller förhöjd kväveoxidhalt i utandningsluften (FeNO), se avsnitt Farmakodynamik, som är otillräckligt kontrollerad trots hög dos inhalerad kortikosteroid (ICS) i kombination med ett annat läkemedel för underhållsbehandling.
Dupixent är indicerat för barn 6 till 11 år, som tillägg till underhållsbehandling vid svår astma med typ 2 inflammation, som kännetecknas av förhöjda nivåer av blod eosinofiler och/eller förhöjd kväveoxidhalt i utandningsluften (FeNO), se avsnitt Farmakodynamik, som är otillräckligt kontrollerad trots medel till hög dos inhalerad kortikosteroid (ICS) i kombination med ett annat läkemedel för underhållsbehandling.
Se övriga indikationer i den obligatoriska informationen.
Verkningsmekanism
MOD
IL-4 OCH IL-13 ÄR CENTRALA DRIVKRAFTER BAKOM TYP 2-INFLAMMATION2,11
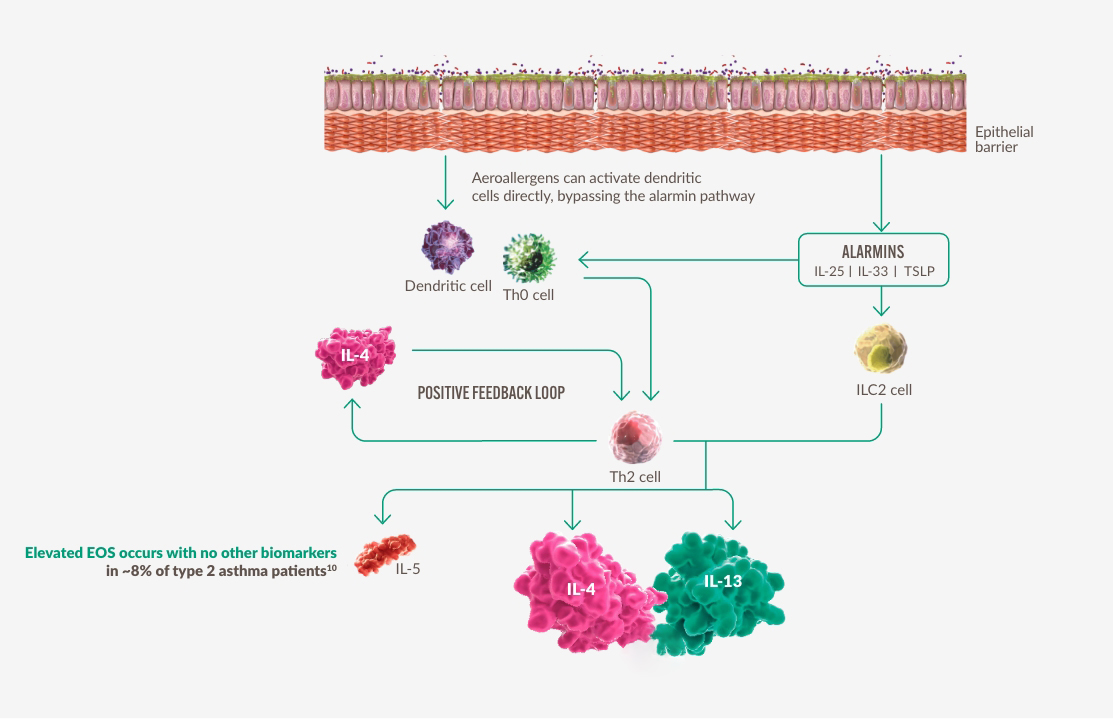
Figure constructed by Sanofi based on ref. 2 and 11.
IL-4 och IL-13 bidrar till systemisk eosinofil och allergisk inflammation samt lokala inflammatoriska effekter2,11
MOA
ÖKAD PRECISION FÖR ATT RIKTA IN SIG PÅ BÅDE IL-4 OCH IL-13 FÖR ATT PÅVERKA TYP 2-INFLAMMATION VID KÄLLAN1,3
SYSTEMIC INFLAMMATIONReduces Type 2 Biomarkers1,2,11-17 |
SYSTEMIC INFLAMMATIONImproves Airway Structures1,2,11-17 |
|
Allergic inflammationa
Eosinophilic inflammationa
IL-5 plays a role in eosinophilic inflammation |
|
DUPIXENT går längre än att bara påverka EOS genom att specifikt rikta in sig på IL-4 och IL-13 för att skydda mot strukturella luftvägsskador och sjukdomsprogression1-3
a Systemisk eosinofil och allergisk inflammation samt lokal luftvägsinflammation.
Patient typer
FLER MÖJLIGHETER FÖR PATIENTER MED TYP 2-ASTMA1
Patientprofilerna är representativa och avser inte verkliga patienter. Individuella resultat kan variera.
DUPIXENT GÅR LÄNGRE ÄN EOS FÖR ATT GÖRA MER FÖR PATIENTER MED TYP 2-ASTMA3,n

IDENTIFIERA TYP 2-ASTMA: FÖRHÖJDA EOS OCH/ELLER FÖRHÖJT FeNO, OAVSETT ALLERGISTATUS5
up to

75% reduction in annualized severe exacerbations5,a

IMPROVED LUNG FUNCTION, WHICH WAS STRONGLY CORRELATED WITH A REDUCTION OF MUCUS PLUGGING related to the anti-inflammatory effect of Dupixent7,m
~3 years
of sustained breathing relief patients can feel18,b
90%
of patients experienced zero exacerbations19,c
Typ 2 biomarkörer
59%
av patienter med typ 2-astma har mer än en förhöjd typ 2-biomarkör10,*
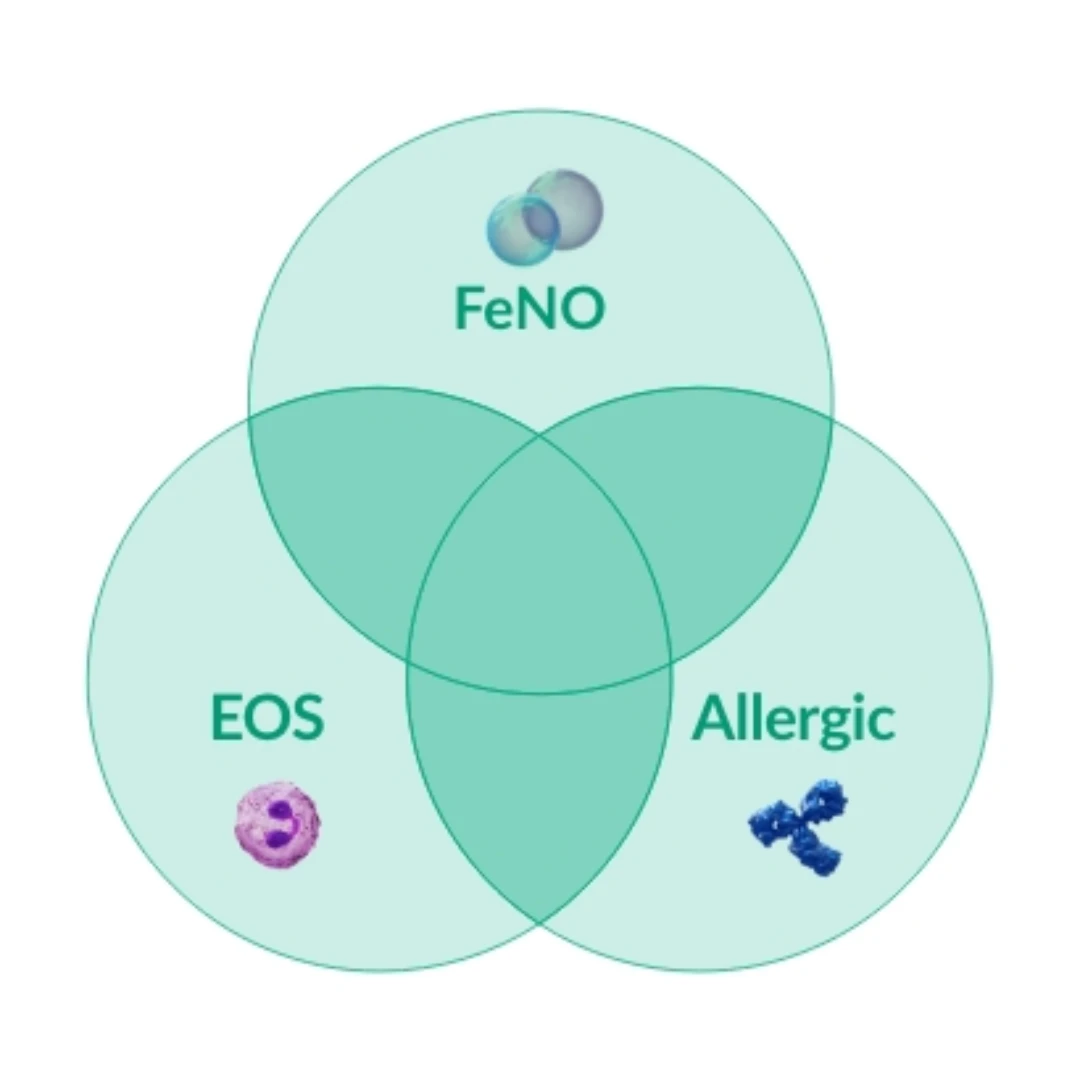
Figure created by Sanofi based on ref. 10
Förhöjda EOS förekommer utan andra biomarkörer hos ~8% av patienter med typ 2-astma10*
* Within the international Severe Asthma Registry, (ISAR) data were analyzed in adults with severe asthma with available biomarkers (n=1,175) from 10 countries in North America, Europe, and Asia, with respecified thresholds for biomarker positivity— (serum IgE ≥75 IU/mL, blood eosinophils ≥300 cells/μL, and FeNO ≥25 ppb); and with hierarchical cluster analysis using biomarkers as continuous variables. Distinct clusters were shown with significant overlap of biomarker positivity in severe asthma.
DUPIXENT GÖR MER FÖR TYP 2-ASTMAPATIENTER SOM ANVÄNDER OCS19

DEN FÖRSTA OCS-KUREN ÄR DET FÖRSTA TECKNET PÅ ATT UTVÄRDERA FÖR TYP 2-ASTMA1,3,19-21

94% of patients maintained complete elimination19,d
up to

250 mL improvement in lung function19,e

90% were exacerbation-free19,f
DUPIXENT GÖR MER FÖR PATIENTER MED ALLERGISK STATUS5

DUPIXENT ÄR EFFEKTIVT HOS PATIENTER MED ALLERGISK STATUS SOM HAR FÖRHÖJDA EOS OCH/ELLER FÖRHÖJT FeNO5
EOS + Allergic

470 mL improvement in lung function5,g
FeNO + Allergic

470 mL improvement in lung function5,h
EOS + FeNO + Allergic

550 mL improvement in lung function5,i
DUPIXENT HOS BARN MED TYP 2-ASTMA20,21

IDENTIFIERA TYP 2-ASTMA: FÖRHÖJDA EOS OCH/ELLER FÖRHÖJT FeNO, OAVSETT ALLERGISTATUS
up to

470 mL improvement in lung function20,j

66% reduction in exacerbation20,k
ZERO
exacerbations in 91% of children21,l
a 75% reduction in annualized severe exacerbations at Week 52 with DUPIXENT 200/300 mg + SOC (n=91) in patients with EOS ≥300, FeNO ≥25, and allergic asthma on high-dose ICS. Only descriptive statistics (no P-values) are available for data due to the single-arm nature of the open-label extension study.
b As observed across a 52-week trial (QUEST) and a 96-week, open-label extension trial (TRAVERSE) of adult and adolescent patients. Improvements in FEV1 observed mean change changes from baseline dupilumab-dupilumab arm (0,35L (SD 0,45) first 52 weeks and 0,38L (SD 0,55) after 100 weeks and 0,35L(SD 0,48) after 148 weeks). Only descriptive statistics (no P-values) available due to open label extension study.
c 89% of patients from QUEST experienced zero exacerbations in Year 3 (Weeks 48-96) when treated with DUPIXENT 200 mg/300 mg Q2W + SOC (n=816) for 52 weeks in Trial 2 (QUEST) and continued with DUPIXENT 300 mg Q2W + SOC in the OLE period (other endpoint). Only descriptive statics (no P-values) are available due to single arm, open label extension study.
d At Week 24 of VENTURE, 48 of 90 in the DUPIXENT/DUPIXENT patient group (53.3%) and 29 of 97 in the placebo/DUPIXENT patient group (29.9%) were free of OCS treatment. Of these, 31 of 33 in the DUPIXENT/DUPIXENT patient group and 21 of 21 in the placebo/DUPIXENT patient group with available OCS dose data remained free of OCS treatment at TRAVERSE Week 48. 14 of 14 in the DUPIXENT/DUPIXENT patient group and 9 of 9 in the placebo/DUPIXENT patient group continued to be free of OCS treatment at TRAVERSE Week 96. Only descriptive statistics (no p value available) due to the single arm nature of the open label extension study.
e 250 mL (+/-0,46L) improvement in FEV1 at week 96 of TRAVERSE from VENTURE baseline in the DUPIXENT/DUPIXENT patient group (n=28). Only descriptive statistics (no P-values availably), due to the single-arm nature of the open-label extension study.
f Of the 67 DUPIXENT/DUPIXENT patients who were treated up to 96 weeks in TRAVERSE, 90% were exacerbation-free during Weeks 48 through 96 of TRAVERSE. Only descriptive statics (no P-values) are available due to single arm, open label extension study.
g 470 mL improvement in FEV1 at Week 52 with DUPIXENT 200/300 mg + SOC (n=141) vs 170 mL with placebo + SOC (n=93) (LSM difference, 290 mL [95% CI: 180, 400 mL]) in patients with EOS ≥300 and allergic asthma on high-dose ICS.
h 470 mL improvement in FEV1 at Week 52 with DUPIXENT 200/300 mg + SOC (n=165) vs 180 mL with placebo + SOC (n=99) (LSM difference, 290 mL [95% CI: 180, 400 mL]) in patients with FeNO ≥25 and allergic asthma on high-dose ICS.
i 550 mL improvement in FEV1 at Week 52 with DUPIXENT 200/300 mg + SOC (n=91) vs 210 mL with placebo + SOC (n=64) (LSM difference, 340 mL [95% CI: 200, 470 mL]) in patients with EOS ≥300, FeNO ≥25, and allergic asthma on high-dose ICS.
j 470 mL improvement in FEV1 at Week 52 with DUPIXENT 100/200 mg + SOC (n=123) vs 240 mL with placebo + SOC (n=53) (LSM difference, 220 mL [95% CI: 110, 330 mL]) in pediatric patients with FeNO ≥20 and allergic asthma on medium- to high-dose ICS.
k 66% reduction in annualized severe exacerbations at Week 52 with DUPIXENT 100/200 mg + SOC (n=114) vs placebo + SOC (n=52) (0.190 vs 0.599) (P=0.0003) in pediatric patients with EOS ≥300 and FeNO ≥20 on medium- to high-dose ICS.
l Most children (286 [91%] of 315) remained exacerbation-free throughout the study. Open-label extension study. Descriptive statistics, no p-value available.
m Study indicate that Dupixent reduced airway inflammation and mucus plugging, and improved airway volume and flow, corresponding to improved lung function and asthma control (prebronchodilator FEV1, P nominal <0,001 and ACQ/Asthma Control Questionnaire P nominal <0,001) for uncontrolled, moderate-to-severe type 2 asthma (EOS ≥300 cells/µL and FeNO ≥25 parts per billion [ppb]) being treated with medium-dose to high-dose ICS.
n Dupixent reduce exacerbations, reduced symptoms, improve lung function, decrease or withdrawal of OGs, irrespective of blood eosinophils count at baseline. Review article, only descriptive.
Effekt
Lung funktion (>1 biomarkörer, FeNO, EOS)
>1 Biomarkör
BÄTTRE ANDNING INNEBÄR FLER MÖJLIGHETER FÖR PATIENTER MED TYP 2-ASTMA5
I en post hoc-analys uppvisade patienter med mer än en förhöjd biomarkör signifikant förbättring av FEV1 vid vecka 525
EOS + Allergic

440 mL improvement5,a
EOS + FeNO

530 mL improvement5,b
FeNO + Allergic

440 mL improvement5,c
EOS + FeNO + Allergic

500 mL improvement5,d

Patientprofilerna är representativa och avser inte verkliga patienter. Individuella resultat kan variera.

VISSA PATIENTER UPPNÅDDE MER ÄN EN HALV LITERS FÖRBÄTTRING AV LUNGFUNKTIONEN5
a 440 mL improvement in pre-BD FEV1 from baseline at Year 3 with DUPIXENT 300 mg Q2W (n=51) in patients with EOS ≥300 and allergic asthma on high-dose ICS.
b 530 mL improvement in pre-BD FEV1 from baseline at Year 3 with DUPIXENT 300 mg Q2W (n=70) in patients with EOS ≥300 and FeNO ≥25 on high-dose ICS.
c 440 mL improvement in pre-BD FEV1 from baseline at Year 3 with DUPIXENT 300 mg Q2W (n=57) in patients with FeNO ≥25 and allergic asthma on high-dose ICS.
d 500 mL improvement in pre-BD FEV1 from baseline at Year 3 with DUPIXENT 300 mg Q2W (n=34) in patients with EOS ≥300, FeNO ≥25 and allergic asthma on high-dose ICS.
a-d Single-arm, open-label extension study - descriptive statistics available (no p-value).
Förhöjd FeNO
BÄTTRE ANDNING INNEBÄR FLER MÖJLIGHETER FÖR TYP 2-ASTMAPATIENTER MED FÖRHÖJT FeNO18,22
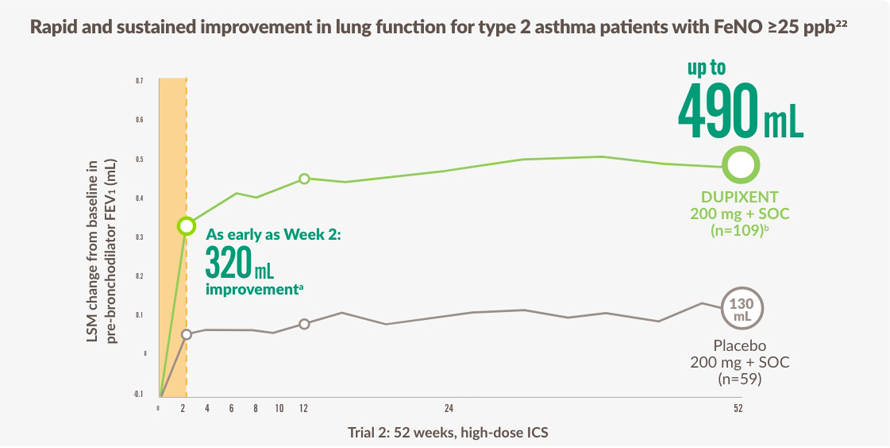
The figure is reproduced by Sanofi based on ref. 22 figure 2.
~3 YEARS OF SUSTAINED RELIEF was seen in the OLE study18,c

ENLIGT GINA ÄR FÖRHÖJT FeNO STARKT PREDIKTIVT FÖR BEHANDLINGSSVAR MED DUPIXENT23
a Primary endpoint Var vecka 12.
b LSM Skillnader, 360 mL (95% CI: 230, 480 mL).
c Enligt observationer från en 52-veckors studie (QUEST) och en 96-veckors öppen förlängningsstudie (TRAVERSE) med vuxna och ungdomar.
Förhöjd EOS
BÄTTRE ANDNING INNEBÄR FLER MÖJLIGHETER FÖR PATIENTER MED FÖRHÖJDA EOS22
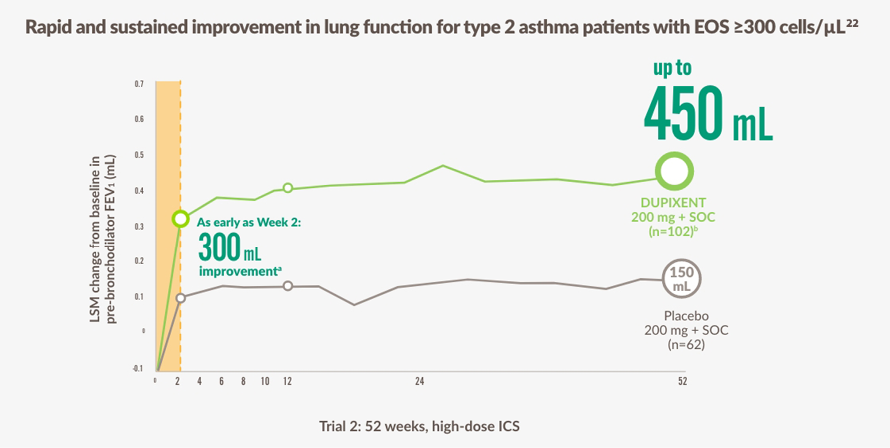
The figure is reproduced by Sanofi based on reference 22, figure 2.
~3 YEARS OF SUSTAINED RELIEF was seen in the OLE study18,c
ENLIGT GINA ÄR FÖRHÖJDA EOS STARKT PREDIKTIVA FÖR BEHANDLINGSSVAR MED DUPIXENT23
a Primärt effektmått var vecka 12.
b LSM-skillnad, 300 mL (95% KI: 180, 410 mL).
c Enligt observationer från en 52-veckors studie (QUEST) och en 96-veckors öppen förlängningsstudie (TRAVERSE) med vuxna och ungdomar.
In patients with EOS ≥500 cells/μL

490 mL improvement in FEV1 at Week 525,a
a 490 mL improvement in pre-BD FEV1 from baseline at Week 52 with DUPIXENT 300 mg Q2W (n=115) in patients with EOS ≥500 on high-dose ICS.
Exacerbations (>1 biomarker, FeNO, EOS)
>1 biomarker
ÖKAD FRIHET FRÅN EXACERBATIONER HOS PATIENTER MED TYP 2-ASTMA5
I en post hoc-analys uppvisade patienter med mer än en förhöjd biomarkör signifikant minskning av exacerbationer vid vecka 525
EOS + Allergic

59% reduction5,a
EOS + FeNO

73% reduction5,b
FeNO + Allergic

64% reduction5,c
EOS + FeNO + Allergic

75% reduction5,d

Patientprofilerna är representativa och avser inte verkliga patienter. Individuella resultat kan variera.
a 59% reduction in annualized severe exacerbations at Week 52 with DUPIXENT 200/300 mg + SOC (n=141) vs placebo + SOC (n=93) (0.270 vs 0.612) (P<0.0001) in patients with EOS ≥300 and allergic asthma on high-dose ICS.
b 73% reduction in annualized severe exacerbations at Week 52 with DUPIXENT 200/300 mg + SOC (n=180) vs placebo + SOC (n=104) (0.189 vs 0.396) (P<0.0001) in patients with EOS ≥300 and FeNO ≥25 on high-dose ICS.
c 64% reduction in annualized severe exacerbations at Week 52 with DUPIXENT 200/300 mg + SOC (n=165) vs placebo + SOC (n=99) and (0.241 vs 0.534) (P<0.0001) in patients with FeNO ≥25 and allergic asthma on high-dose ICS.
d 75% reduction in annualized severe exacerbations at Week 52 with DUPIXENT 200/300 mg + SOC (n=91) vs placebo + SOC (n=64) (0.152 vs 0.412) (P<0.0001) in patients with EOS ≥300, FeNO ≥25, and allergic asthma on high-dose ICS.
Förhöjd FeNO
ÖKAD FRIHET FRÅN EXACERBATIONER FÖR PATIENTER MED FÖRHÖJT FeNO18,22
Signifikant minskning av exacerbationer för patienter med typ 2-astma med FeNO ≥25 ppb vid vecka 52, bibehållen i ~3 år18,22

Patient profiles are representative and are not actual patients. Individual results may vary.
up to

69% reduction24,a
~3 YEARS OF SUSTAINED PROTECTION was seen in the OLE study18,b

ENLIGT GINA ÄR FÖRHÖJT FeNO STARKT PREDIKTIVT FÖR BEHANDLINGSSVAR MED DUPIXENT23
a In annualized severe exacerbations at Week 52 (QUEST) with DUPIXENT 200 mg + SOC (n=145) vs placebo + SOC (n=78) (0.40 vs 1.30) (P<0.001), and 55% reduction with DUPIXENT 300 mg + SOC (n=159) vs placebo + SOC (n=89) (0.57 vs 1.26) (P<0.001).
b As observed across a 52-week trial (QUEST) and a 96-week, open-label extension trial (TRAVERSE) of adult and adolescent patients.
A severe exacerbation event was defined as a deterioration of asthma requiring the use of systemic corticosteroids for ≥3 days or hospitalization or an ED visit. The population in dupilumab asthma trials included patients on medium- to high-dose ICS. All data presented above represent the high-dose ICS population.
Förhöjd EOS
ÖKAD FRIHET FRÅN EXACERBATIONER FÖR PATIENTER MED FÖRHÖJDA EOS22
Signifikant minskning av exacerbationer för patienter med typ 2-astma med förhöjda EOS vid baslinjen vid vecka 52, bibehållen i ~3 år22

Patientprofilerna är representativa och avser inte verkliga patienter. Individuella resultat kan variera.
EOS ≥150 cells/μL
up to

55% Reduction22,a
EOS ≥300 cells/μL
up to

66% Reduction22,b
EOS ≥500 cells/μL
up to

70% Reduction5,c
~3 YEARS OF SUSTAINED PROTECTION was seen in the OLE study18,d

ENLIGT GINA ÄR FÖRHÖJDA EOS STARKT PREDIKTIVA FÖR BEHANDLINGSSVAR MED DUPIXENT23
a In annualized severe exacerbations at Week 52 with DUPIXENT 200 mg + SOC (n=223) vs placebo + SOC (n=126) (0.56 vs 1.24) (P<0.001), and up to 53% reduction with DUPIXENT 300 mg + SOC (n=228) vs placebo + SOC (n=127) (0.53 vs 1.15) (P<0.001).
b In annualized severe exacerbations at Week 52 with DUPIXENT 300 mg + SOC (n=144) vs placebo + SOC (n=79) (0.51 vs 1.48) (P<0.001), and up to 59% reduction with DUPIXENT 200 mg + SOC (n=128) vs placebo + SOC (n=80) (0.55 vs 1.35) (P<0.001).
c As observed across a 52-week trial (QUEST) and a 96-week, open-label extension trial (TRAVERSE) of adult and adolescent patients. Only statistic available (no p-value).
d As observed across a 52-week trial (QUEST) and a 96-week, open-label extension trial (TRAVERSE) of adult and adolescent patients. Only statistic available (no p-value).
A severe exacerbation event was defined as a deterioration of asthma requiring the use of systemic corticosteroids for ≥3 days or hospitalization or an ED visit.
The population in dupilumab asthma trials included patients on medium- to high-dose ICS. All data presented above represent the high-dose ICS population.
QUEST22 was a randomized, double-blind, placebo-controlled, parallel-group trial that assessed the efficacy of DUPIXENT in 1,902 patients 12 years of age or older patients with uncontrolled, moderate-to-severe asthma. The primary endpoints were the annualized rate of severe asthma exacerbations and the absolute change from baseline to Week 12 in FEV1 before bronchodilator use in the overall trial population. Secondary endpoints included the exacerbation rate and FEV1 in patients with a blood eosinophil count of 300 or more per cubic millimeter. Asthma control and DUPIXENT safety were also assessed.
TRAVERSE5 was an open-label extension study that assessed the safety and efficacy of DUPIXENT 300 mg every 2 weeks up to 96 weeks in adults and adolescents (aged 12- 84 years) with moderate-to-severe or oral-corticosteroid–dependent severe asthma who had completed a previous DUPIXENT asthma study (including QUEST). The primary endpoint was the number and percentage of patients with any treatment-emergent adverse events. Secondary endpoints included an annualized exacerbation rate (AER) over the treatment period and change from parent study baseline in pre-bronchodilator FEV1.
OCS-eliminering (24 veckor, 49-58 veckor)
24 Veckor
DUPIXENT GÖR MER FÖR ATT eliminera24

Patient profiles are representative and are not actual patients. Individual results may vary.
52% of patients completely eliminated OCS by Week 24 in the VENTURE study1,19,24
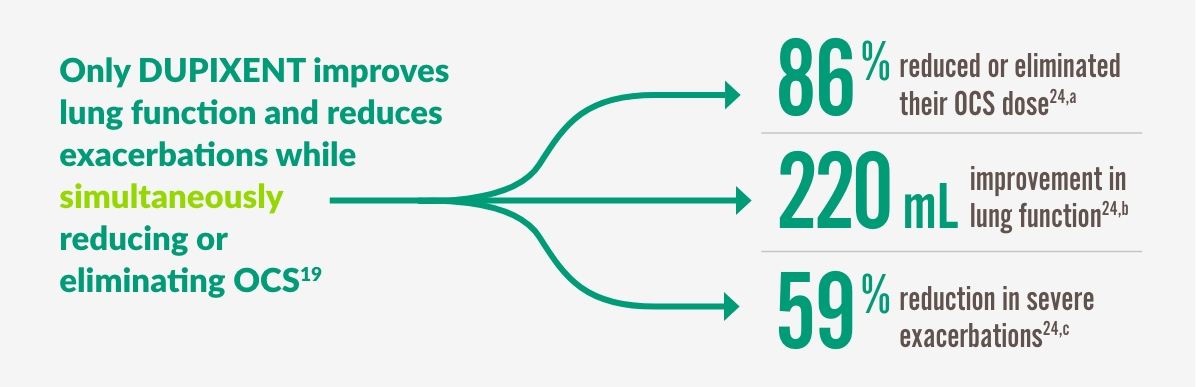
a 86% of patients reduced or eliminated OCS use by Week 24 with DUPIXENT 300 mg + SOC (n=103) vs 68% with placebo + SOC (n=107) (P<0.001).
b 220 mL improvement in pre-bronchodilator FEV1 at Week 24 with DUPIXENT 300 mg + SOC (n=103) vs 10 mL with placebo + SOC (n=107) (LSM difference, 220 mL [95% CI: 90, 340 mL]).
c 59% reduction in annualized rate of severe exacerbations at Week 24 with DUPIXENT 300 mg + SOC (n=103) vs placebo + SOC (n=107) (0.65 vs 1.60; rate ratio: 0.41 [95% CI: 0.26, 0.63]).
49-58 weeks
DUPIXENT GÖR MER FÖR ATT SÄKERSTÄLLA ATT PATIENTER FÖRBLIR FRIA FRÅN OCS19

Patient profiles are representative and are not actual patients. Individual results may vary.
Complete elimination of OCS achieved through Week 24 and sustained through Week 9619
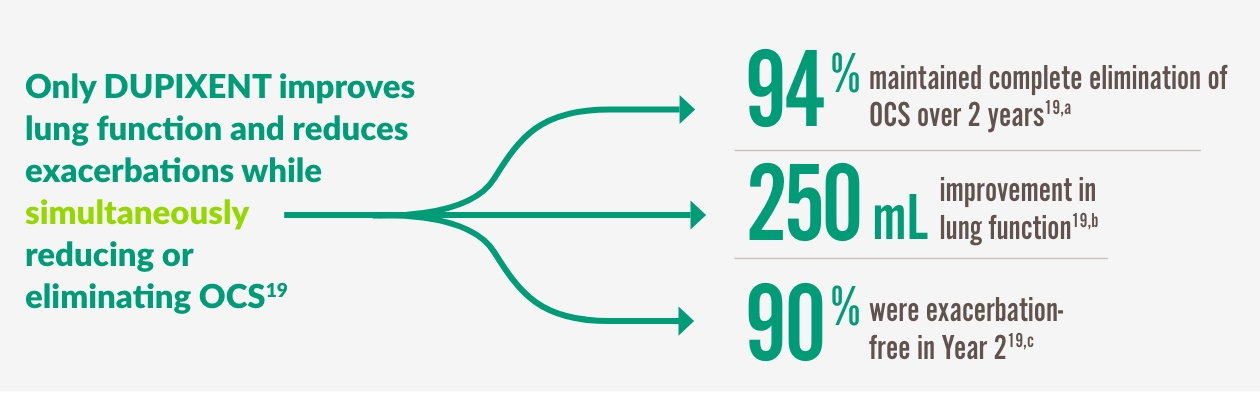
a 86% of patients reduced or eliminated OCS use by Week 24 with DUPIXENT 300 mg + SOC (n=103) vs 68% with placebo + SOC (n=107) (P<0.001).
b 220 mL improvement in pre-bronchodilator FEV1 at Week 24 with DUPIXENT 300 mg + SOC (n=103) vs 10 mL with placebo + SOC (n=107) (LSM difference, 220 mL [95% CI: 90, 340 mL]).
c 59% reduction in annualized rate of severe exacerbations at Week 24 with DUPIXENT 300 mg + SOC (n=103) vs placebo + SOC (n=107) (0.65 vs 1.60; rate ratio: 0.41 [95% CI: 0.26, 0.63]).
Mukusproppar
Dupixent och mukusproppar4,6
|
Lung function improvement, was strongly correlated with a reduction of mucus plugging4 |
||
|
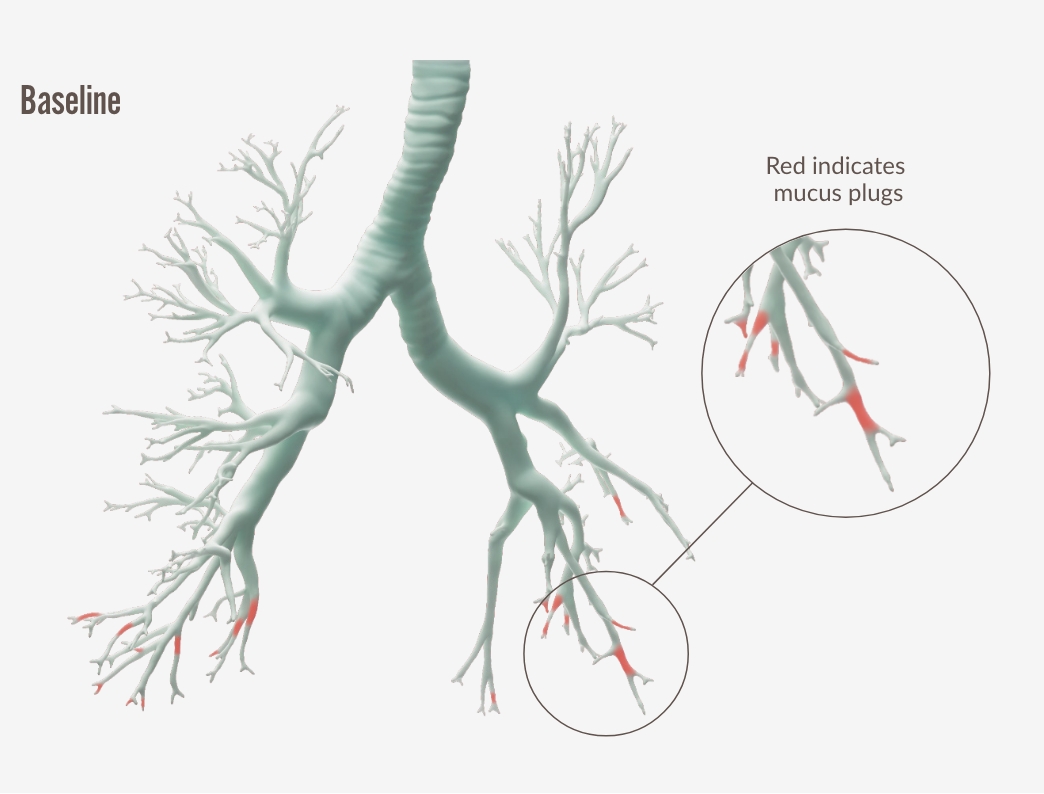
Individual images correspond to patients with the median change in the high- dose ICS population. Adopted by Sanofi based on ref. 4, figure S8.
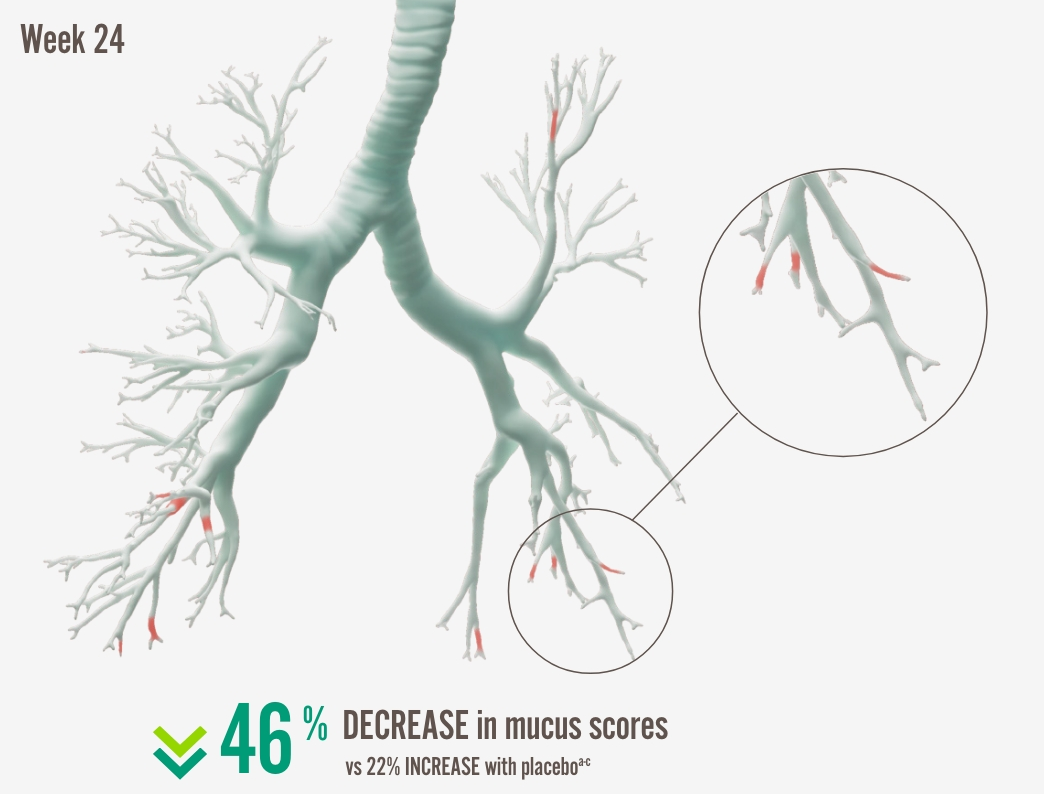
Individual images correspond to patients with the median change in the high-dose ICS population.
7 Based on Poster(34) presented at the International Conference of the American Thoracic Society. May 17-22. San Diego, CA
a In the high-dose ICS population, 3.8 decrease in global mucus score at Week 24 from baseline score of 8.3 with DUPIXENT 300 mg + SOC (n=34) vs 1.5 increase from baseline score of 6.9 with placebo + SOC (n=21) (LSM difference: -5.3 [95% CI: - 7.704, -2.821], nominal P<0.0001).
b Mucus scores range from 0 to 20, with higher scores indicating airway occlusion. Scores between 0.5 to 3.5 indicate low mucus levels, and scores between 4 and 20 indicate high mucus levels.26
c In the ITT population, 3.48 decrease in global mucus score at Week 24 from baseline score of 7.2 with DUPIXENT 300 mg + SOC (n=72) vs 1.44 increase from baseline score of 6.9 with placebo + SOC (n=37) (LSM difference: -4.92 [0.80]), nominal P<0.001.
d Pearson’s correlation coefficient for mucus score/pre-BD FEV1: r[60]=-0.61, nominal P<0.001.
e LS mean change in ACQ-7 score from baseline at Week 24: -1.4 from 2.9 at baseline with DUPIXENT + SOC (n=72) vs -0.6 from 3.0 at baseline with placebo + SOC (n=37) (LSM difference vs placebo: -0.7 [95% CI: -1.1, -0.4]; nominal P<0.001).
The VESTIGE trial was a phase 4, randomized, double-blind, placebo-controlled study that used functional respiratory imaging (FRI) to evaluate the effect of dupilumab on airway inflammation, as well as structural and functional changes in the lungs. The primary endpoints were proportion of patients achieving FeNO <25 ppb at Week 24, and change from baseline to Week 24 in airway volume. Secondary endpoints included change in global mucus score from baseline at Week 24 determined by high-resolution computed tomography (HRCT), and change from baseline in airway resistance.
ACQ-5 and AQLQ
DUPIXENT GÖR MER FÖR ATT FÖRBÄTTRA ASTMAKONTROLL OCH LIVSKVALITET1
Signifikant förbättrade ACQ-5- och AQLQ(S)-poäng hos patienter med typ 2-astma vid vecka 521,a

Patient profiles are representative and are not actual patients. Individual results may vary.
ACQ-5
up to

76% of patients improved asthma control1,b
AQLQ(S)
up to

71% of patients improved QoL1,c
a The responder rate was defined as an improvement in core of 0.5 or more (scale range 0-6 for ACQ-5 and 1-7 for AQLQ[S]).
b 76% ACQ-5 responders within ACQ-5 with DUPIXENT 300 mg + SOC (n=277) vs 64% with placebo + SOC (n=159), and 74% with DUPIXENT 200 mg + SOC (n=262) vs 65% with placebo + SOC (n=141) in patients on medium-to-high dose ICS with FeNO ≥25.
c 71% AQLQ(S) responders with DUPIXENT 300 mg + SOC (n=239) vs 55% with placebo + SOC (n=124), and 65% with DUPIXENT 300 mg + SOC (n=248) vs 55% with placebo + SOC (n=129) in patients on medium-to-high dose ICS with EOS ≥300.
DUPIXENT förbättrade ACQ-5-poäng signifikant1
QUEST was a 52-week randomized, double-blind, placebo-controlled, parallel-group trial that assessed the efficacy of DUPIXENT in 1,902 patients (12 years of age or older) with uncontrolled, moderate-to-severe asthma on a medium to high dose of ICS and a second controller (+/- a third controller). Subjects were randomized to receive either 200 mg or 300 mg DUPIXENT Q2W (or matching placebo for either 200 mg or 300 mg). following an initial dose of 400 mg, 600 mg, or placebo, respectively. The primary endpoints were the annualized rate of severe asthma exacerbations during the 52-week period and the absolute change from baseline to Week 12 in pre-bronchodilator FEV1 in the overall population. Secondary endpoints included the annualized exacerbation rate and pre-BD FEV1 in patients with a blood eosinophil count of 300 or more per microliter, as well as responder rates in the ACQ-5 and AQLQ(S), and safety.1
Studieupplägg (ungdomar/vuxna, barn)
adolescents/adults
DUPIXENT HAR STUDERATS I ETT KLINISKT PROGRAM SOM INKLUDERADE ~3 000 PATIENTER1,22,24,28
Kliniska prövningar inkluderade patienter (12+) med okontrollerad, måttlig till svår astma driven av typ 21,22,24,28
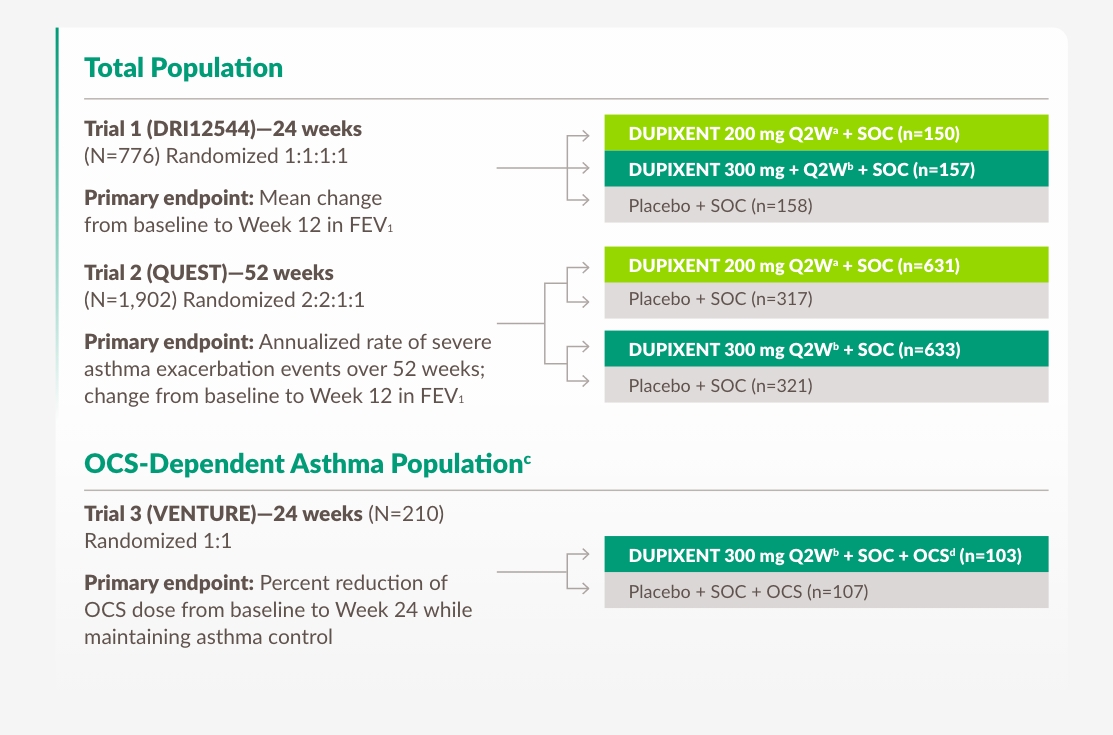
a With 400 mg loading dose.
b With 600 mg loading dose.
c OCS-dependent patients required daily oral corticosteroids, in addition to regular use of high-dose–inhaled corticosteroids plus an additional controller.
d OCS dose was reduced every 4 weeks during the OCS reduction phase (Weeks 4-20), as long as asthma control was maintained.
Egenskaper hos vuxna och ungdomspatienter i 3 studier1,22,24,28
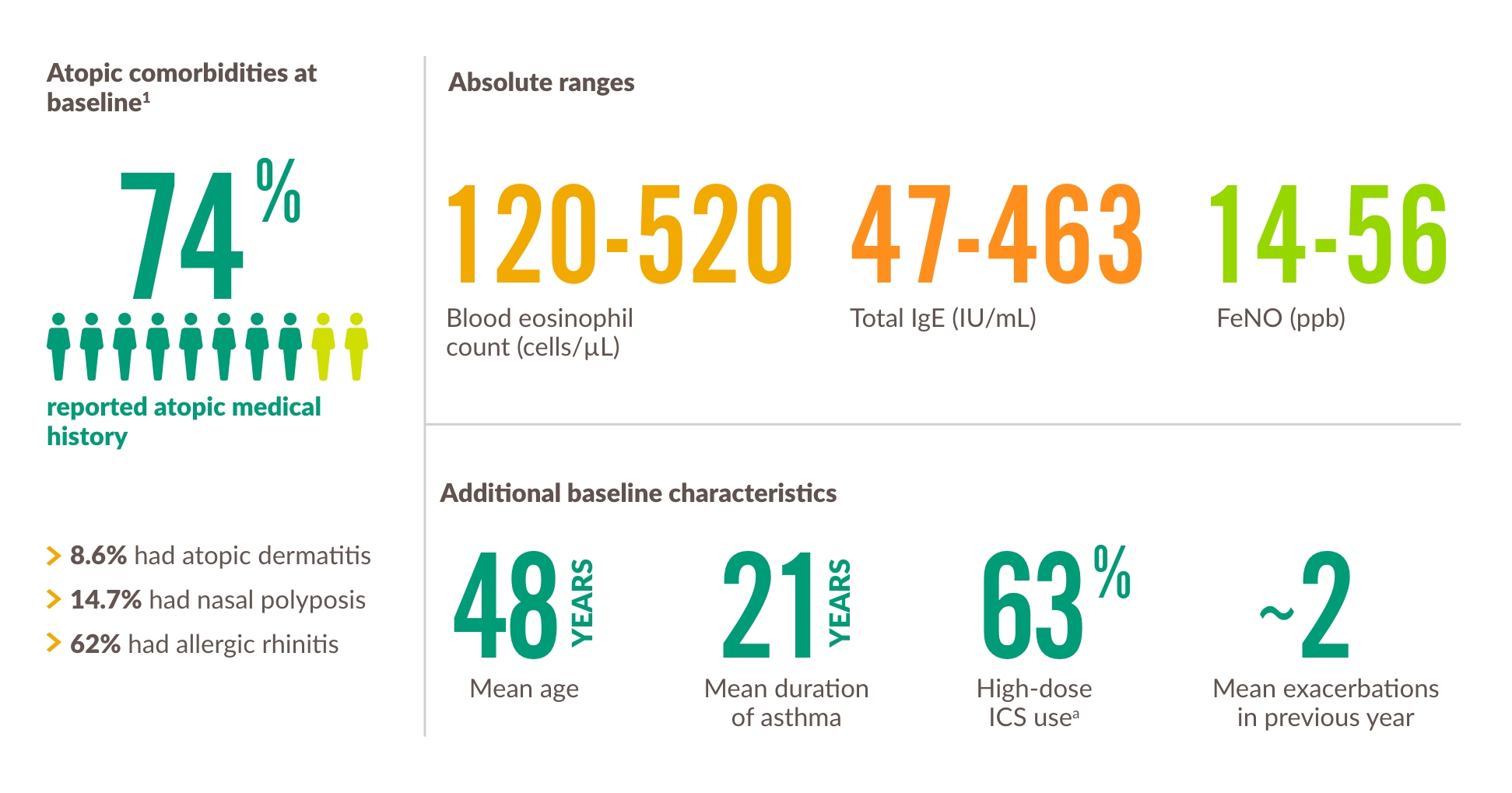
a High-dose ICS use across the 3 trials was 49.5% in DRI12544, 51.5% in QUEST, and 88.6% in VENTURE. The population in dupilumab asthma trials included patients on medium- to high-dose ICS. The medium ICS dose was defined as equal to 500 mcg fluticasone or equivalent per day.
Extension trial of patients from DRI12544, QUEST, and VENTURE1,18,19
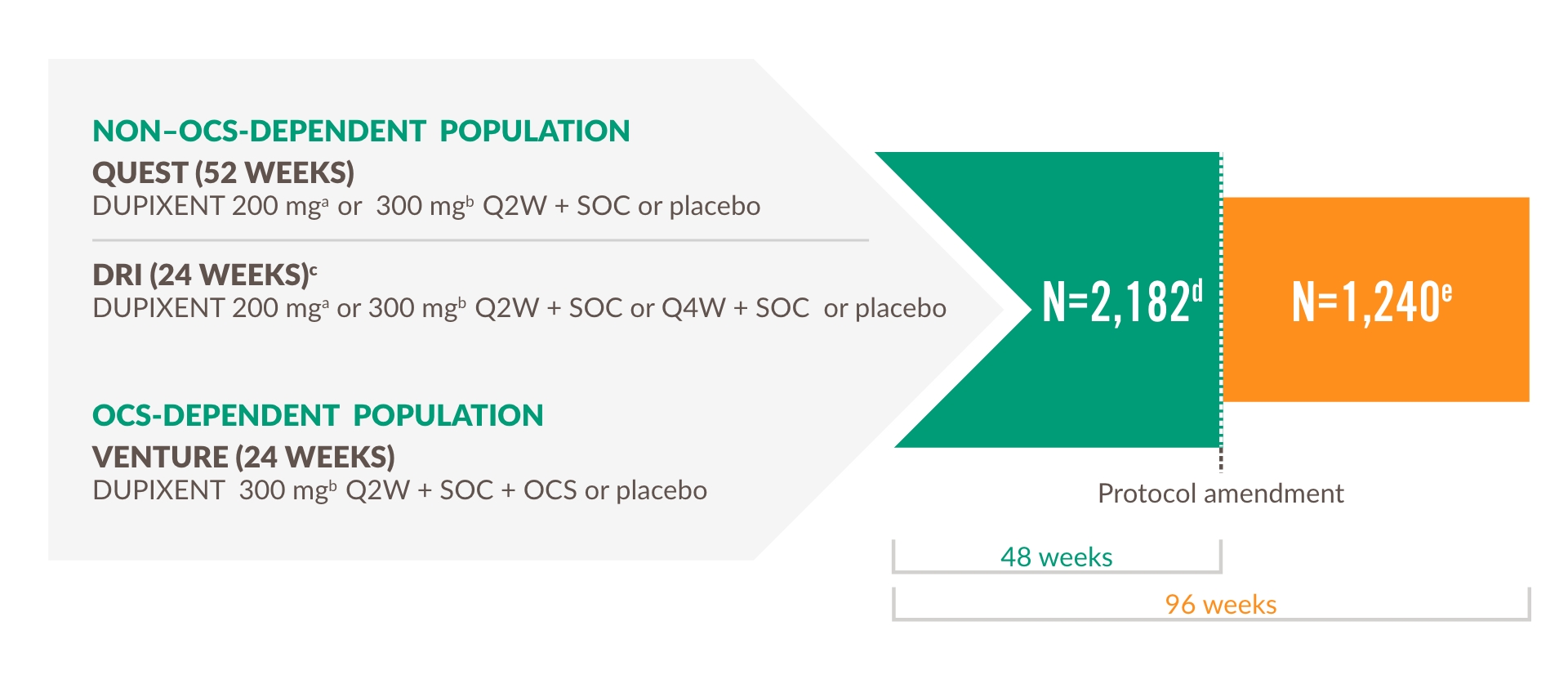
Primary endpoint
The number and percentage of patients with any treatment-emergent adverse events.
The LIBERTY ASTHMA TRAVERSE evaluated the long-term safety, tolerability, and efficacy of DUPIXENT in adults and adolescents who enrolled from a previous DUPIXENT study, including DRI12544, QUEST, and VENTURE.19
Select other endpoints
- Number and annualized rate of severe exacerbation events
- Improvement in FEV1
- In the OCS-dependent population, percent reduction from baseline in OCS dose and proportions of patients achieving ≥50% reduction and completely tapering off OCS19
a With 400 mg loading dose.
b With 600 mg loading dose.
c It should be noted that eligible patients completed the 16-week, post-treatment, followup period of the parent study before being rolled over into TRAVERSE.
d Total number of subjects enrolled and exposed to treatment in OLE.
e Total number of subjects who continued to be exposed to treatment beyond 48 weeks.
VESTIGE: the largest study using novel FRI technology with an asthma biologic.4
The VESTIGE trial was a phase 4, randomized, double-blind, placebo-controlled study that used functional respiratory imaging (FRI) to evaluate the effect of dupilumab on airway inflammation, as well as structural and functional changes in the lungs. The study population consisted of patients aged 21 to 70 years with uncontrolled moderateto- severe asthma (Asthma Control Questionnaire [ACQ]-5 score ≥1.5), pre-BD FEV1 ≤80% of predicted value, ≥1 exacerbation in prior year, blood eosinophil count ≥300 cells/μL, and FeNO ≥25 ppb.4,a
Primary endpoints:
- Proportion of patients achieving FeNO <25 ppb at Week 24
- Percent change from baseline to Week 24 in airway volume corrected for lung volume (siVaw) at TLC
Secondary endpoints:
- Change in global mucus score from baseline at Week 24 determined by highresolution computed tomography (HRCT)
- Percent change from baseline to Week 24 in airway resistance • (siRaw) at TLC
a FRI (functional respiratory imaging) is a novel computational modeling technique that combines high-resolution CT imaging with computational fluid dynamics to create patient-specific 3D models of lung structure and function. Increase in airway volume and decrease in airway resistance revealed by FRI have been shown to mirror spirometry findings, with the added benefit of illustrating the regional distribution of the effects on lung function.
FULL INDICATION STATEMENTS
ATOPIC DERMATITIS1
DUPIXENT is indicated for the treatment of moderate-to-severe atopic dermatitis in adults and adolescents 12 years and older who are candidates for systemic therapy.
DUPIXENT is indicated for the treatment of severe atopic dermatitis in children 6 to 11 years old who are candidates for systemic therapy.
ASTHMA1
DUPIXENT is indicated in adults and adolescents 12 years and older as add-on maintenance treatment for severe asthma with type 2 inflammation characterised by raised blood eosinophils and/or raised fraction of exhaled nitric oxide (FeNO), who are inadequately controlled with high dose inhaled corticosteroids (ICS) plus another medicinal product for maintenance treatment.
DUPIXENT is indicated in children 6 to 11 years old as add-on maintenance treatment for severe asthma with type 2 inflammation characterised by raised blood eosinophils and/or raised fraction of exhaled nitric oxide (FeNO), who are inadequately controlled with medium to high dose inhaled corticosteroids (ICS) plus another medicinal product for maintenance treatment.
CRS WITH NASAL POLYPS (CRSwNP)1
DUPIXENT is indicated as an add-on therapy with intranasal corticosteroids for the treatment of adults with severe CRSwNP for whom therapy with systemic corticosteroids and/or surgery do not provide adequate disease control.
PRURIGO NODULARIS (PN)1
DUPIXENT is indicated for the treatment of adults with moderate-to-severe prurigo nodularis (PN) who are candidates for systemic therapy.
EOSINOPHILIC ESOPHAGITIS (EoE)1
DUPIXENT is indicated for the treatment of eosinophilic esophagitis in adults and children aged 1 year and older, weighing at least 15 kg, who are inadequately controlled by, are intolerant to, or who are not candidates for conventional medicinal therapy.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD)1
DUPIXENT is indicated in adults as add-on maintenance treatment for uncontrolled chronic obstructive pulmonary disease (COPD) characterised by raised blood eosinophils on a combination of an inhaled corticosteroid (ICS), a long-acting beta2- agonist (LABA), and a long-acting muscarinic antagonist (LAMA), or on a combination of a LABA and a LAMA if ICS is not appropriate.
Säkerhet
Safety
6 ÅRS ETABLERAD SÄKERHET VID ASTMA1
DUPIXENT tolererades genomgående väl hos vuxna och ungdomar med astma, och uppvisade en säkerhetsprofil liknande den som observerats hos pediatriska populationer1,a
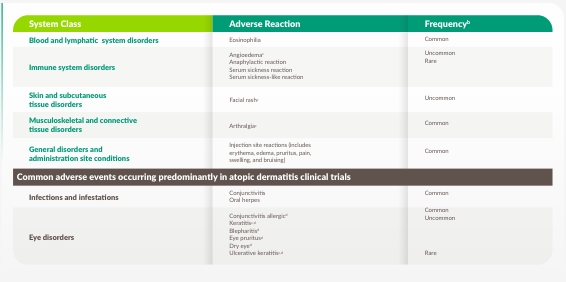
a As observed across 3 randomized, placebo-controlled, multicenter trials of 24 to 52 weeks duration (DRI12544, QUEST, and VENTURE), and a 96-week, open-label extension trial (TRAVERSE) and another 3-year open-label extension study (TRAVERSE CONTINUATION), in adult and adolescent patients. The safety profile observed in the pediatric asthma clinical study (a 52-week multicenter, randomized, double-blind, placebo-controlled study [VOYAGE]) was similar to that seen in adults.
b Common (≥1/100 to <1/10); Uncommon (≥1/1,000 to <1/100); Rare (≥1/10,000 to <1/1,000).
c From postmarketing reporting.
d Eye disorders and oral herpes occurred predominantly in atopic dermatitis studies. The frequencies for eye pruritus, blepharitis, and dry eye were common, and ulcerative keratitis was uncommon in atopic dermatitis studies. In adults with moderate-to-severe atopic dermatitis, the safety and tolerability profile of DUPIXENT has been studied for 4 years in an open-label extension study and was demonstrated to be generally consistent with the 52-week data.
API
ABBREVIATED PRESCRIBING INFORMATION
Dosering
Dosing
PATIENTER ELLER VÅRDGIVARE KAN ADMINISTRERA HEMMA ELLER DU KAN ADMINISTRERA PÅ MOTTAGNINGEN
Rekommenderade doseringsscheman per patienttyp1:
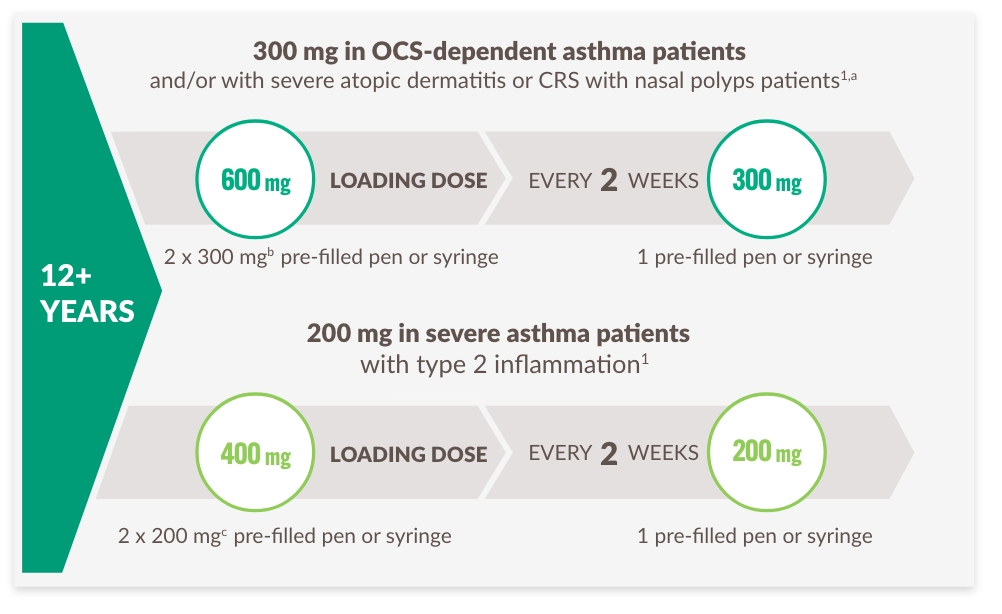
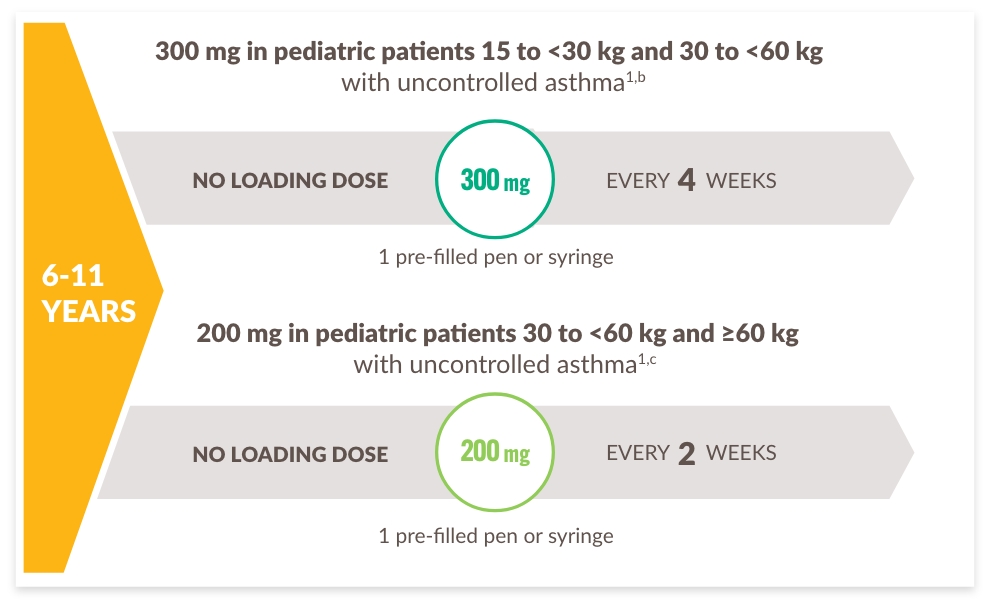
a Advise atopic dermatitis patients with comorbid asthma not to adjust or stop their asthma treatments without consultation with their physicians. For patients with severe asthma and who are on oral corticosteroids, or for patients with severe asthma and comorbid moderate-to-severe atopic dermatitis or adults with comorbid severe chronic rhinosinusitis with nasal polyposis, use an initial dose of 600 mg (two 300 mg injections), followed by 300 mg every other week, which should be administered as a subcutaneous injection.
b 300 mg=2 mL solution.
c 200 mg=1.14 mL solution.
Administration
DUPIXENT Pre-filled Pen1 |
DUPIXENT Pre-filled Syringe1 |
|
|
|
|
|
DUPIXENT is intended for use under the guidance of a healthcare provider1
- DUPIXENT can be injected by a patient or caregiver at home after training in a subcutaneous injection technique using the auto-injection pen (for adults and adolescents) or pre-filled syringe
- Physicians or nurses should provide proper training to patients and/or caregivers on the preparation and administration of DUPIXENT prior to use, according to the Instructions for Use
- DUPIXENT can be administered in the office under the guidance of a healthcare provider if the patient or caregiver is not an appropriate candidate to administer the injection
Resources
ACQ-5, Asthma Control Questionnaire, 5-Item version
ACQ-5-IA, Asthma Control Questionnaire-5, Interviewed Administered
ACQ-7-IA, Asthma Control Questionnaire-7, Interviewer Administered
AQLQ(S), Asthma Quality of Life Questionnaire
BD, bronchodilator
COPD, chronic obstructive pulmonary disease
CRS, chronic rhinosinusitis
CRSwNP, chronic rhinosinusitis with nasal polyps
CSU, chronic spontaneous urticaria
CT, computed tomography
ED, emergency department
EoE, eosinophilic esophagitis
EOS, eosinophils
FeNO, fraction of exhaled nitric oxide
FEV1, forced expiratory volume in 1 second
FEF, Forced Expiratory Flow
FRI, functional respiratory imaging
GINA, Global Initiative for Asthma
HCPs, healthcare professionals
HD-ICS, high-dose inhaled corticosteroids
HRCT, high-resolution computed tomography
ICS, inhaled corticosteroids
ILC2, type 2 innate lymphoid cells
ISAR, International Severe Asthma Registry
ITT, intention-to-treat
LABA, long-acting beta agonist
LAMA, long-acting muscarinic antagonist
LSM, least squares mean
LTRA, leukotriene receptor antagonist
OCS, oral corticosteroids
OLE, open-label extension
OR, odds ratio
PAQLQ(S)-IA, Pediatric Asthma Quality of Life Questionnaire with Standardized Activities-Interviewer Administered
PACQLQ, Pediatric Asthma Caregiver’s Quality of Life Questionnaire
PROs, patient-reported outcomes
PSBL, parent study baseline
Q2W, once every 2 weeks
Q4W, once every 4 weeks
QoL, quality of life
SCS, systemic corticosteroid
SE, standard error
siRaw, airway resistance
siVaw, airway volume
SOC, standard of care
TLC, total lung capacity
TSLP, thymic stromal lymphopoietin
References
-
DUPIXENT Summary of Product Characteristics, 2024.
-
Gandhi NA, Bennett BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35-50. doi:10.1038/nrd4624
-
Brusselle GG, Koppelman GH. Biologic therapies for severe asthma. N Engl J Med. 2022;386(2):157-171. doi:10.1056/NEJMra2032506
-
Castro, M, Papi A, Porsbjerg C, et al. Effect of dupilumab on airway inflammation and mucus plugs measured with functional respiratory imaging: results from a randomised, double-blind, placebo-controlled study in patients with type 2 asthma (VESTIGE). Lancet. Forthcoming 2024
-
Pavord ID, Bourdin A, Papi A, et al. Dupilumab sustains efficacy in patients with moderate-to-severe type 2 asthma regardless of inhaled corticosteroids dose. Allergy. 2023;78(11):2921-2932. doi:10.1111/all.15792
-
Shah PA, Brightling C. Biologics for severe asthma—which, when and why? Respirology. 2023;28:709–721. doi:10.1111/resp.14520
-
Porsjberg C, Dunican EM, Lugogo NI, et al. Effect of dupilumab treatment on mucus plugging and mucus volume in type 2 asthma: the phase 4 VESTIGE trial. Poster presented at the International Conference of the American Thoracic Society (ATS). May 17-22, 2024. San Diego, CA. Poster A34.
-
IQVIA Sanofi Integrated DUPIXENT Platform, data through August 31, 2024.
-
Denton E. Price DB, Tran TN, et al. Cluster analysis of inflammatory biomarker expression in the International Severe Asthma Registry. J Allergy Clin Immunol Pract. 2021;9(7):2680-2688.e7. doi:10.1016/j.jaip.2021.02.059
-
Robinson D, Humbert M, Buhl R, et al. Revisiting type 2-high and type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy. 2017;47(2):161-175. doi:10.1111/cea.12880
-
Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8(3):193-204. doi:10.1038/nri2275
-
Alving K, Malinovschi A. Basic aspects of exhaled nitric oxide. In: European Respiratory Monograph. European Respiratory Society Publications; 2010;49:1-31.
-
Saatian B, Rezaee F, Desando S, et al. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers. 2013;1(2):e24333. doi:10.4161/tisb.24333
-
Manson ML, Säfholm J, James A, et al. IL-13 and IL-4, but not IL-5 nor IL-17A, induce hyperresponsiveness in isolated human small airways. J Allergy Clin Immunol. 2020;145(3):808-817.e2. doi:10.1016/j.jaci.2019.10.037
-
Bonser LR, Zlock L, Finkbeiner W, Erle DJ. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J Clin Invest. 2016;126(6):2367- 2371. doi:10.1172/JCI84910
-
Lee CG, Homer RJ, Zhu Z, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med. 2001;194(6):809-821. doi:10.1084/jem.194.6.809
-
Wechsler ME, Ford LB, Maspero JF, et al. Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): an open-label extension study. Lancet Respir Med. 2022;10(1):11-25. doi:10.1016/S2213- 2600(21)00322-2
-
Sher LD, Wechsler ME, Rabe KF, et al. Dupilumab reduces oral corticosteroid use in patients with corticosteroid-dependent severe asthma: an analysis of the phase 3, open-label extension TRAVERSE trial. Chest. 2022;162(1):46-55. doi:10.1016/j.chest.2022.01.071
-
Maspero JF, Peters AT, Chapman KR, et al. Long-term safety of dupilumab in patients with moderate-to-severe asthma: TRAVERSE Continuation Study. J Allergy Clin Immunol Pract. 2024:12(4)991-997.e6. doi:10.1016/j.jaip.2023.12.043
-
Bacharier LB, Maspero JF, Katelaris CH, et al. Assessment of long-term safety and efficacy of dupilumab in children with asthma (LIBERTY ASTHMA EXCURSION): an open-label extension study. Lancet Respir Med. 2024;12(1):45-54. doi:10.1016/S2213-2600(23)00303-X
-
Bourdin A, Papi AA, Corren J, et al. Dupilumab is effective in type 2-high asthma patients receiving high-dose inhaled corticosteroids at baseline. Allergy. 2021;76(1):269-280. doi:10.1111/all.14611
-
Global Initiative for Asthma. Global strategy for asthma management and prevention, 2024. Accessed June 14, 2024. https://ginasthma.org/wpcontent/uploads/2024/05/GINA-2024-Strategy-Report- 24_05_22.WMS.pdf
-
Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378(26):2475-2485. doi:10.1056/NEJMoa1804093
-
Tang M, Elicker BM, Henry T, et al. Mucus plugs persist in asthma, and changes in mucus plugs associate with changes in airflow over time. Am J Respir Crit Care Med. 2022;205(9):1036-1045. doi:10.1164/rccm.202110-2265OC
-
Porsjberg C, Dunican EM, Lugogo NI, et al. Effect of dupilumab treatment on mucus plugging and mucus volume in type 2 asthma: the phase 4 VESTIGE trial. Supplementary Results, 2024.
-
Castro, M, Papi A, Porsbjerg C, et al. Effect of dupilumab on airway inflammation and mucus plugs measured with functional respiratory imaging: results from a randomised, double-blind, placebo-controlled study in patients with type 2 asthma (VESTIGE). Lancet. Forthcoming 2024.
-
Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-tosevere uncontrolled asthma. N Engl J Med. 2018;378(26):2486-2496. doi:10.1056/NEJMoa1804092
-
Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high dose inhaled corticosteroids plus a long-acting ß2 agonist: a randomised double-blind placebocontrolled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31-44. doi:10.1016/S0140-6736(16)30307-5

© 2024 Sanofi and Regeneron Pharmaceuticals, Inc. All Rights Reserved.
MAT-BE-2500019-v2.0 01/2025